The topics in this section help study administrators set up, integrate, and manage study data. This page also outlines the
study data model, helpful for planning study structure.
Tutorials
Topics
More Topics
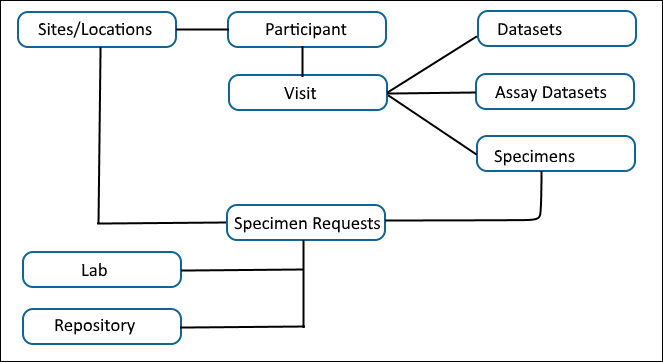
Study Data Model
The core entities of a Study (its "keys") are
Participants (identified by "Participant IDs") and
Time, either Visits (identified by "Visit IDs" or "SequenceNums") or Dates.
Participants appear at planned locations (Sites) at expected points in time (Visits) for data collection. At such Visits, scientific personnel collect Datasets (including Clinical and Assay Datasets) and may also collect specimen samples. This data can all be uploaded or linked to the Study.
A Study also tracks and manages Specimen Requests from Labs, plus the initial delivery of Specimens from Sites to the Specimen Repository.