The Web Configuration Portal supports a
Data Sharing Consent workflow for capturing the participant's willingness to share data with with third parties for research. This is in addition to the primary consent they provide to the organization conducting the study.
There is a separate documentation site for the FDA COVID-19 MyStudies app, with information about using that application for the electronic consent portion of this functionality.
When in use, the mobile app needs to provide the collected flag value to the Response server, and then the Response server needs to store the provided value, associate it with that participant’s record, and provide the value to analysts when they view or retrieve response data. Note that the Response server simply conveys the value of the flag; it has no ability to enforce behavior. The onus is on the analyst to take the appropriate action (e.g., filtering on the flag column, sharing data or not).
Functional Design
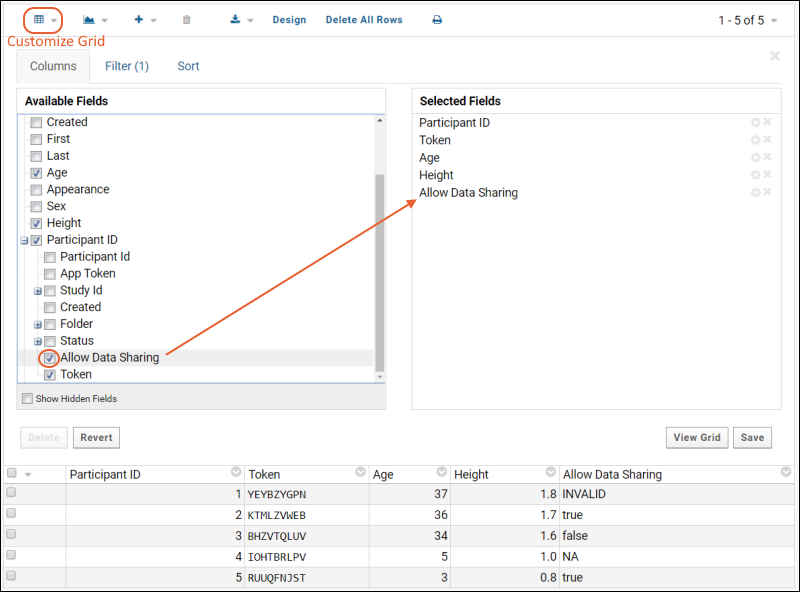
The Response server stores the allowDataSharing flag values in the column: mobileappstudy.Participant.AllowDataSharing. The Participant table is already available in the response lists, so this new column is also available for adding to grids via Customize Grid.
Checking the box for "Allow Data Sharing" in Customize Grid makes that column available in the grid for viewing, filtering, sorting, export, API querying, etc. LabKey SQL queries can also use this column.
Related Topics
