To create a new study, you need to be logged in with administrator permissions.
Create the Study Container
First we create an empty container for the study data to live in. We recommend creating studies inside subfolders, not directly inside of projects.
- Navigate to a project, in which you will create a subfolder.
- Create an empty "study" type subfolder:
- Go to Admin > Folder > Management. Click the button Create Subfolder.
- Enter a Name for the study, choose the folder type Study, and click Next.
- On the Users/Permissions page, click Finish. (You can configure permissions later by selecting Admin > Folder > Permissions.)
Create or Import the Study
You are now on the overview tab of your new study folder. You have the option to create a study from scratch, or importing an pre-existing study.
Set Study Properties
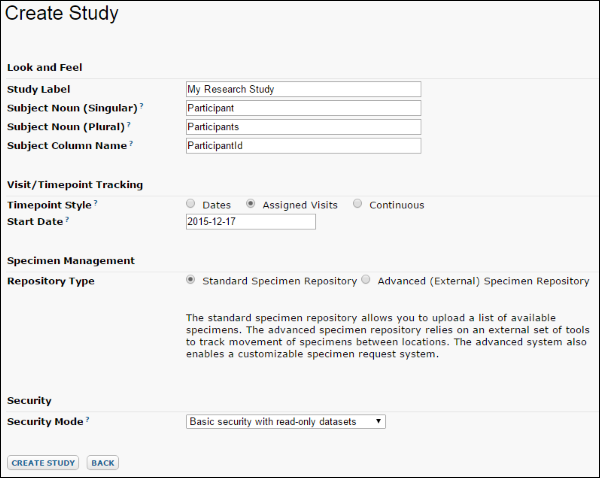
- Look and Feel: Name your study and choose how to refer to subjects.
- Visit/Timepoint Tracking: Choose whether your study tracks time by date or visit. See Continuous Studies for studies which use neither.
- Specimen Management: Declare the type of specimen repository you will use.
- Security: Select basic or custom security and whether to allow edits to datasets.
When you are finished, click the
Create Study button to create a study in your new project or folder.
If you would like to create additional custom study properties, you can do so at the project level. A project administrator can define properties that are available to all studies within the project. For more information, see
Custom Study Properties.
Design a Vaccine Study
A vaccine study is specialized to collect data about specific vaccine protocols, associated immunogens, and adjuvants. A team can agree upon the necessary study elements in advance and design a study which can be used as a template to create additional studies with the same pre-defined study products, treatments, and expected schedule, if desired. This can be particularly helpful when your workflow includes study registration by another group after the study design is completed and approved.
Once you have created a study, whether directly or via import, you can standardize shared vaccine study features among multiple studies.
[
Video Overview: Study Designer - treatment data and assay schedule]
Related Topics