This page takes you on a tour through the tabs and features in our online
Interactive Example Study to highlight some key features of a LabKey Study. One application of a study is translational research where complex clinical datasets are integrated with outcomes in order to identify treatment protocols that produce better outcomes for specific patients. All the data within this interactive example is fictional and for demonstration purposes only.
Collaborative Research Dashboard
Within a study folder, tabs and subfolders can be used to encapsulate functional sets of the tools available. You can define custom tabs and place tools (webparts) on them as desired, or use the default set of study tabs:
- Overview: title, abstract, protocol, and other basic info.
- Participants: clinical and demographic data for participants, cohorts and groups.
- Clinical and Assay Data: browse available datasets, reports, and visualizations.
- Specimen Data: specimen request and tracking system.
- Manage (visible to admins only): a dashboard of project properties.
See a similar
interactive example.
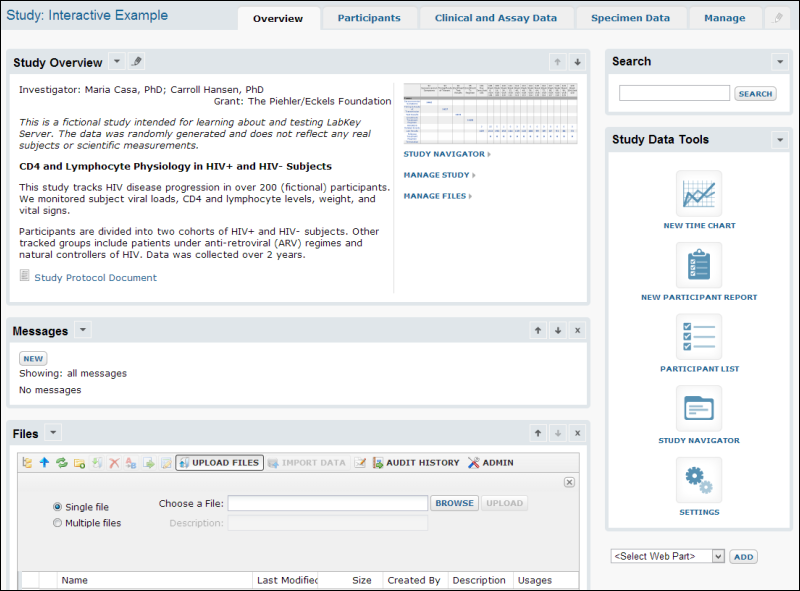
Overview
The
Study Overview webpart displays the investigator, grant, description, and other customizable
Study Properties you define.
The
Study Navigator helps you track the status of each part of the study. It shows how much data is available for each dataset at each point in time. Time in a study can be organized by visit or by date-based timepoints which can either be fixed relative to the study start data or based on individual participant start dates.
Search
The ability to search across all aspects of the data is one of the most powerful features of a LabKey Study. Data from clinic visits, lab results, and instrument-derived assay data can be integrated in ways that support discovery of relationships and trends that can be connected to give a broad vision of how to improve treatment protocols. You can also focus on particular pieces of data in a complete way.
Study-wide search is supported from the Overview tab: For example, if you enter a phrase or even a participantID such as 249318596, you will see links to all documents and datasets that include reference to that participant. Context-specific searches are available as well, such as for
all the whole blood specimens available for a given participant.
Participants
Study participants can be grouped by cohort, demographic information, or other groups you define. Hovering over a label will highlight cohort members on the participant list.
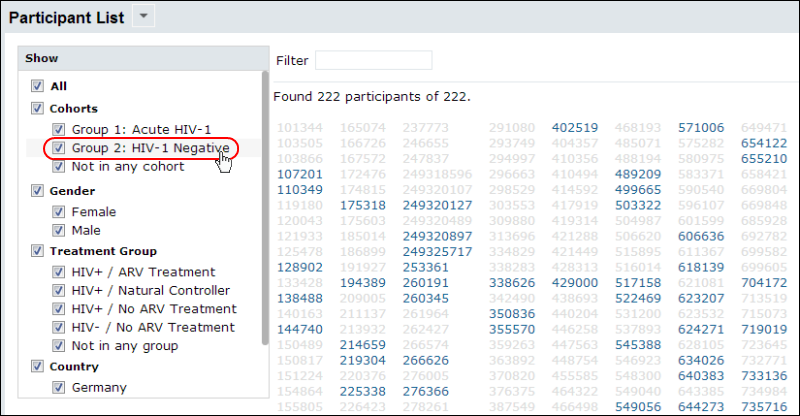
Custom participant views can be defined to show relevant pieces of data including charts and graphs that are dynamically updated as new data is integrated.
Clicking a participantID will open the details page for that individual. You may also create a
Participant Detail web part for a specific participant of interest to display that detail on any portal page.
Clinical and Assay Data
A study dataset is like a list that has been rationalized with participantIDs and dates. Dataset views can be customized to integrate columns from different sources and fields can be customized with validators, lookups, and other programmatic quality control features.
Visualizations
Graphs and charts, like the following time chart and scatter plot, can be created using LabKey Server's data visualization tools. Customize to show the specific measures and participants or cohorts that best illustrate your research.
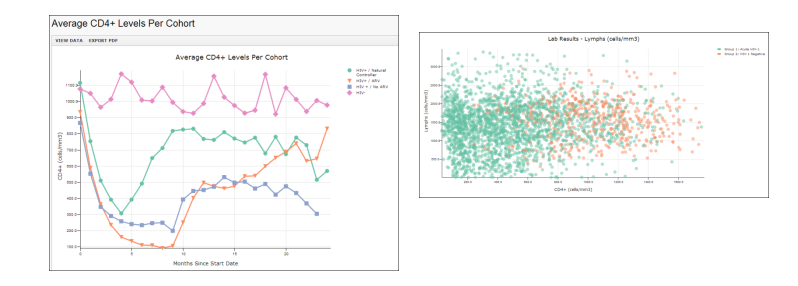 Browse the list of datasets and charts in the interactive example
Browse the list of datasets and charts in the interactive example.
Specimen Data
LabKey Server provides configurable tools for managing specimen data and request systems. For more information, start here:
Specimen Tracking. You can also explore some of the features in our live
interactive example.
Manage
The Study administrator uses the tools and options on the
Manage tab to track and control many aspects of the study. The
Study Schedule offers a broad view of progress and current status:
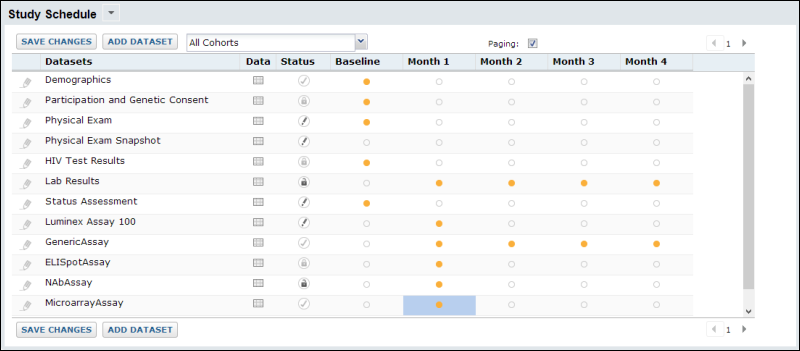
Among the available options, study reloading can be configured to automatically incorporate new data from an external source on a regular schedule, participant groups and cohorts can be defined, security and schedules can be defined and tracked. Protected health information can be obscured in a demonstration mode or aliased to offer the ability to share data and results without compromising privacy of study partipants. Studies can also be exported, imported, published, and ancillary studies can be created with selected participants and datasets from the source study.
Related Topics
The following tutorials give step-by-step instructions for building the Demo Study:
 Custom participant views can be defined to show relevant pieces of data including charts and graphs that are dynamically updated as new data is integrated.Clicking a participantID will open the details page for that individual. You may also create a Participant Detail web part for a specific participant of interest to display that detail on any portal page.
Custom participant views can be defined to show relevant pieces of data including charts and graphs that are dynamically updated as new data is integrated.Clicking a participantID will open the details page for that individual. You may also create a Participant Detail web part for a specific participant of interest to display that detail on any portal page.
 Browse the list of datasets and charts in the interactive example.
Browse the list of datasets and charts in the interactive example.