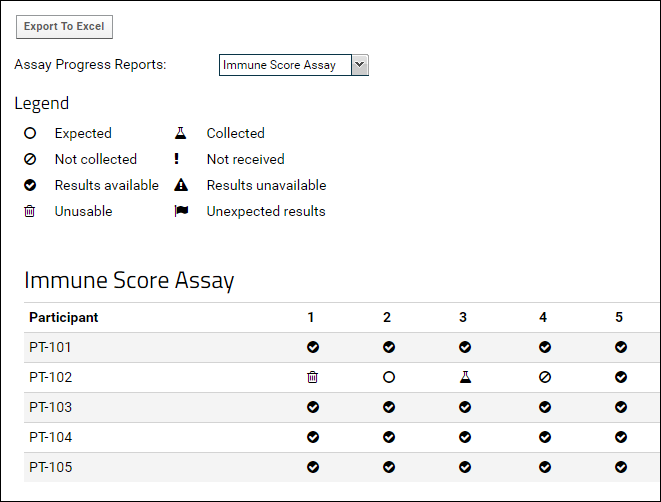
The assay progress report is designed to present an overview of the assay processing lifecycle in a study. When samples or specimens are collected and a series of assays need to be run on a specific schedule, you can use this report to see at a glance, for each participant/timepoint:
- whether the samples have been collected or not,
- whether assay results are available or not, and
- whether any adverse events have occurred, such as unusable or unexpected assay results
For example the status report below shows that most samples have been successfully processed resulting in valid assay data, but one participant (PT-102) has an incomplete specimen processing history.
Topics
Data Requirements
Assay progress reports are only available in a study folder. The following data sources are used to render the assay progress report:
| Data Source | Required? | Description | Source Schema and Table(s) |
|---|
| Participants | Required | The study must have participant data present. | study.Participant |
| Visits | Required | Study visits must be defined. Both date-based or visit-based studies are supported. | study.Visit |
| Assay Schedule | Required | Assay schedules must be defined, as described in the topic Manage Assay Schedule. | study.AssaySpecimen
study.AssaySpecimenVisit |
| Assay results | Required | Some table must be present to hold assay results. This can be a dataset or an assay design that has been linked to the study. | User defined assay results. |
| Status Information Query | Optional (see below for details) | Status information is drawn from a query. Any query, such as a query based on a list, can serve this function as long as it has the required fields. | User defined query. |
Status Information Query
The query to determine sample status must expose the following fields:
- ParticipantId (of type String)
- VisitId (of type Integer)
- SequenceNum (of type Double)
- Status (of type String)
Allowed values for the Status field are listed below. Allowed values are case-sensitive.
- expected
- collected
- not-collected
- not-received
- available
- not-available
- unusable
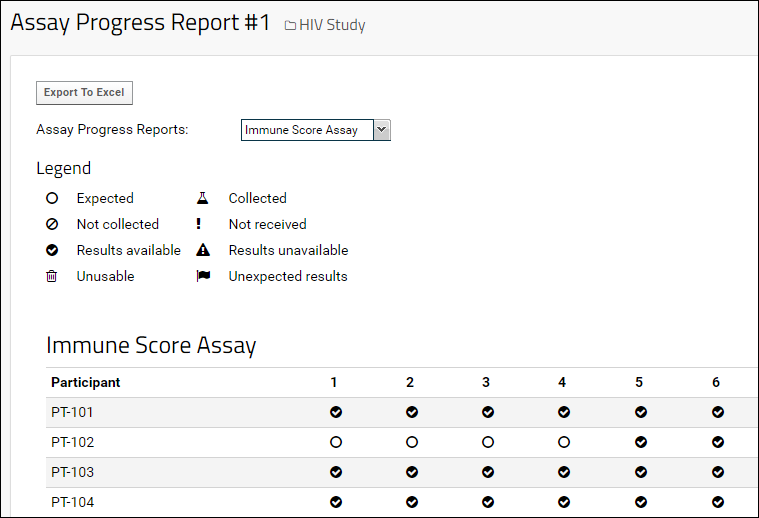
Note that the
Status Information Query is not strictly necessary to render a report. If the query is omitted, a simple report can be rendered based on the assay results in the study: if assay results are present for a given Participant/Visit, then the report will display the status "available" (
); otherwise "expected" (
) will be displayed, as shown below.
Create the Assay Progress Report
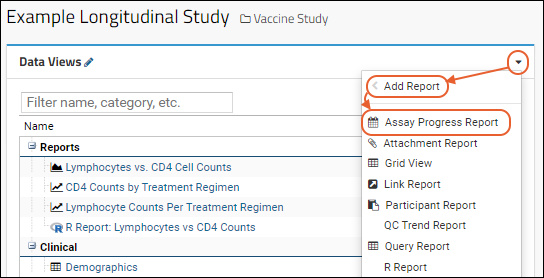
Once the data sources are in place, you can add a progress report for any of your assays.
- Click the Clinical and Assay Data tab.
- On the Data Views web part, click the dropdown triangle () and select Add Report > Assay Progress Report
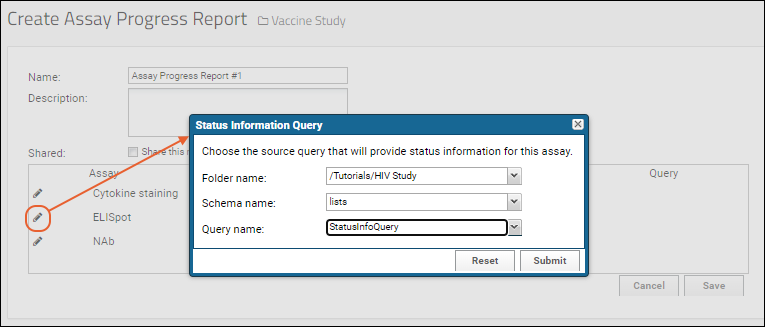
- For each assay you wish to associate with a Status Information Query, click the icon.
- In the popup, use the dropdowns to point to the query which contains the status information, in this case a list named "SpecimenStatus", and click Submit.
- To make the report visible to others who can view this folder, check Share this report with all users?. Leaving this checkmark unselected makes it visible only to you and site admins.
- To finish the progress report, click Save
- The progress report is added to the Data Views web part, or via (Admin) > Manage Views. You could also add it to a study tab using the Report web part.
Export to Excel
The
Export to Excel button is activated when a single assay is selected for display. Clicking downloads all of the assay data for the selected assay.

Related Topics




